Abstract
Background: Among many causes of thrombocytopenia acquired during hospitalization, heparin-induced thrombocytopenia (HIT), an immune complex-mediated thrombogenic state with high morbidity and mortality, is one of the most feared etiologies. The clinical '4T' score is an established method to assess risk for HIT and guide immediate management(Lo, Juhl et al. 2006). However, the 4T score is not consistently used by clinicians, leading to potential over-testing and suboptimal management of suspected HIT. Our objective in this Quality Improvement (QI) project was to implement a mandatory 4T calculator (4TC) into the electronic medical record (EMR) to improve the positive predictive value of HIT antibody (HITA) testing, decrease the incidence of testing of low-risk patients, and to improve clinical management of suspected HIT.
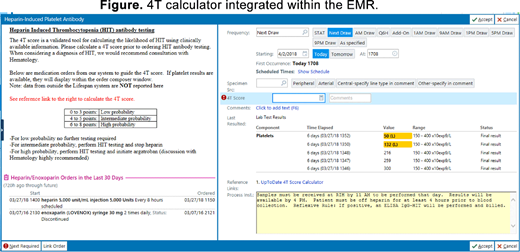
Methods: We developed a 4TC that assisted clinicians by integrating EMR-derived data on heparin exposure and the five most recent platelet counts with a link to an online calculator as well as clinical suggestions (Figure). As a part of this QI project, clinicians were required to enter the calculated 4T score before ordering a polyspecific HITA assay, which serves as the screening test for HIT in our institution. We reviewed laboratory records for all HITA tests performed over a six-month period, including 3 months before and after the implementation of a "hard stop" for HITA order in the EMR. We extracted data on the ordering hospital service team, 4T score documented in notes (if any), as well as quality of care indicators including rates of clinically recommended heparin cessation and initiation of alternative anticoagulation in the subgroup of patients with intermediate or high 4T score at the time of HITA order. In this QI project, we refrained from any statistical testing and summarized pre-specified outcome measures: incidence of HITA testing, positive predictive value of the HITA assay, and quality of care indicators.
Results: We identified 99 (53 prior to the 4TC and 46 post) cases of HITA orders in the study period, among which there were 4 cases of confirmed HIT (either with positive IgG or positive serotonin-release assay [SRA]). Three HIT cases occurred after the 4TC was implemented. After the implementation of the 4TC, the positive predictive value for the HITA assay increased from 9% to 21%. The rate of clinically appropriate heparin order discontinuation increased from 72% to 88%, as did the initiation of alternative anticoagulation from 14% to 27%.
HITA assay was most commonly ordered by the internal medicine service (35% of tests), non-cardiac intensive care service (30%), coronary care and cardiothoracic surgery services (21%), neurology (5%), and general surgery/other services (9%). Implementation of the 4TC did not significantly change this distribution, but the overall incidence of HITA orders decreased from 4.0 to 3.4 per 1000 admissions. We observed a high frequency of confirmatory SRA testing requested (18%), which is the reflex confirmatory test performed at our institution, even in cases of negative polyspecific HITA or HIT IgG.
Conclusions: Addition of a 4T score calculator into the EMR which provides immediate feedback on patient's prior heparin exposure and recent platelet counts allows clinicians to consistently use the 4T score when considering HIT. Importantly, it also helps to educate non-hematologists on the proper management of HIT. Improved predictive value of the HITA assay suggests that our 4TC led to ordering HITA in a more appropriate patient population. We also demonstrated improved quality of care for suspected HIT, with increased rates of heparin cessation and initiation of alternative anticoagulation in patients with intermediate or high pre-test probability. Identifying clinical teams that order a disproportionate number of HITA assays will help us to direct education to improve clinical management. This QI project has led to other areas of improvement, particularly with regard to rational ordering of the SRA. At only three months of review since implementation of the 4TC, we have noted an improvement in the management of suspected HIT and a slight reduction in the incidence of testing. With continued review, we hope to demonstrate further improvement, as practitioners continue to use the 4TC and rely on clinical rationale rather than reflexive laboratory testing when evaluating thrombocytopenia in the hospital.
Olszewski:TG Therapeutics: Research Funding; Spectrum Pharmaceuticals: Consultancy, Research Funding; Genentech: Research Funding. Reagan:Alexion: Honoraria; Takeda Oncology: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.